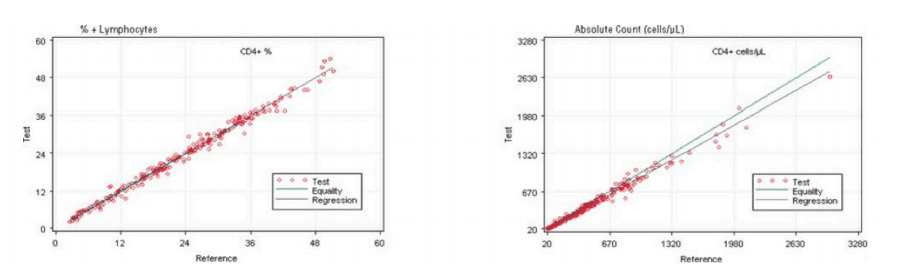
Comparing the AQUIOS CL PLG Application to the FC500 MCL FlowCARE PLG Application
The performance of the AQUIOS PLG System (or application) was assessed by comparing the results to the comparator estimation of difference as described in CLSI EP9-A3 Measurement Procedure Comparison and Bias Estimation Using Patient Samples; Approved Guideline, Third Edition. The comparator used FlowCARE PLG CD4 Reagent, ImmunoPrep Lysing reagent, and Flow-Count for CD4+ cell enumeration on FC 500 MCL flow cytometer with CXP software. The method comparison is comprised of HIV specimens from 38 pediatric and 202 adult subjects. The data provided in Table 1 and Figure 1 supports the premise that the systems are equivalent in their performance enumerating CD4+ lymphocytes in peripheral whole blood. Values are expressed in terms of percentage (%) of the total lymphocyte count and as absolute count (cells/µL) for total CD4+ cells.
Table 1. Measurement Procedure Comparison - Whole Blood
Table 2. Measurement Procedure Comparison of the AQUIOS PLG Application and the FC500 MCL FlowCARE PLG
Figure 1. Regression Analysis of CD4+ Cells on the AQUIOS PLG Application and the Predicate
Conclusions
Based on this study, the data suggests a strong correlation between the AQUIOS CL PLG application and the FC500 MCL FlowCARE PLG application.
AQUIOS CL is a Class I Laser Product
Purity and recovery are important in the calculation of percentages and absolute counts of lymphocyte subsets. Fill the form to download our gating strategy for Tetra reagents.

HIV Advanced Disease Management

HIV Advanced Disease Management Solutions
Monitoring CD4 lymphocyte counts is essential in providing critical information that impacts patient care. CD4 monitoring allows caregivers to know when the disease transforms and stage its progression so they can implement the most appropriate intervention..
Fast Throughput Subset Analysis with AQUIOS CL
In the clinical management of immune deficiency diseases, accurately counting the absolute cell numbers of leukocyte subsets and measuring the percentage of individual subtypes in blood is critical.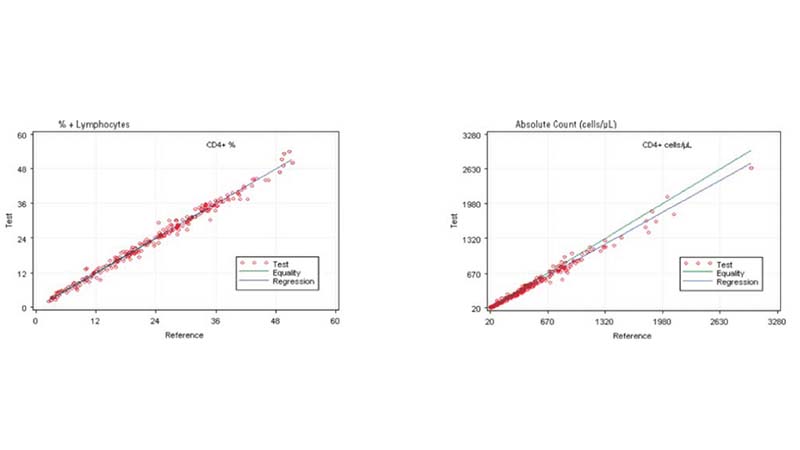
Comparing the AQUIOS CL PLG Application to the FC500 MCL FlowCARE PLG Application
Based on this study, the data suggests a strong correlation between the AQUIOS CL PLG application and the FC500 MCL FlowCARE PLG application.
AQUIOS CL Tetra and Aged Whole Blood samples
Based on this study, the AQUIOS CL instrument with Tetra application provides accurate results for recovery of the T, B and NK lymphocyte subsets in clinical and normal specimens collected into the EDTA K3 tubes when stored at room temperature for up to 24 hours.Error Prevention on AQUIOS CL overview
Laboratory standards and regulations set forth by governmental bodies such as the Centers for Disease Control (CDC) help to define the procedures that laboratories follow
Error prevention during Startup and Worklist creation
The AQUIOS CL system employs several features that work to prevent errors during startup, cleaning, and worklist generation, including Autocleaning, Test Requests to and from the LIS and Creating Worklists.
Error Prevention During Sample Prep
The AQUIOS CL system employs several features that work to prevent errors during sample prep.