ICH Q2 Validation of Analytical Procedures for Pharmaceutical TOC Analyzers
The tripartite International Conference on Harmonization (ICH) Q2 guide on validation of analytical procedures is not new, but it can give very good advice when validating laboratory Total Organic Carbon (TOC) analyzers for pharmaceutical use, especially when a user is considering changing analyzer brands or TOC analysis technologies. This webinar discusses how ICH Q2 can be applied to TOC analyzers to ensure they are suitable for pharmaceutical applications.
Watch the webinar to learn
- About the elements of ICH Q2.
- How ICH Q2 can be applied to laboratory TOC analyzers to ensure they are suitable for pharmaceutical applications.
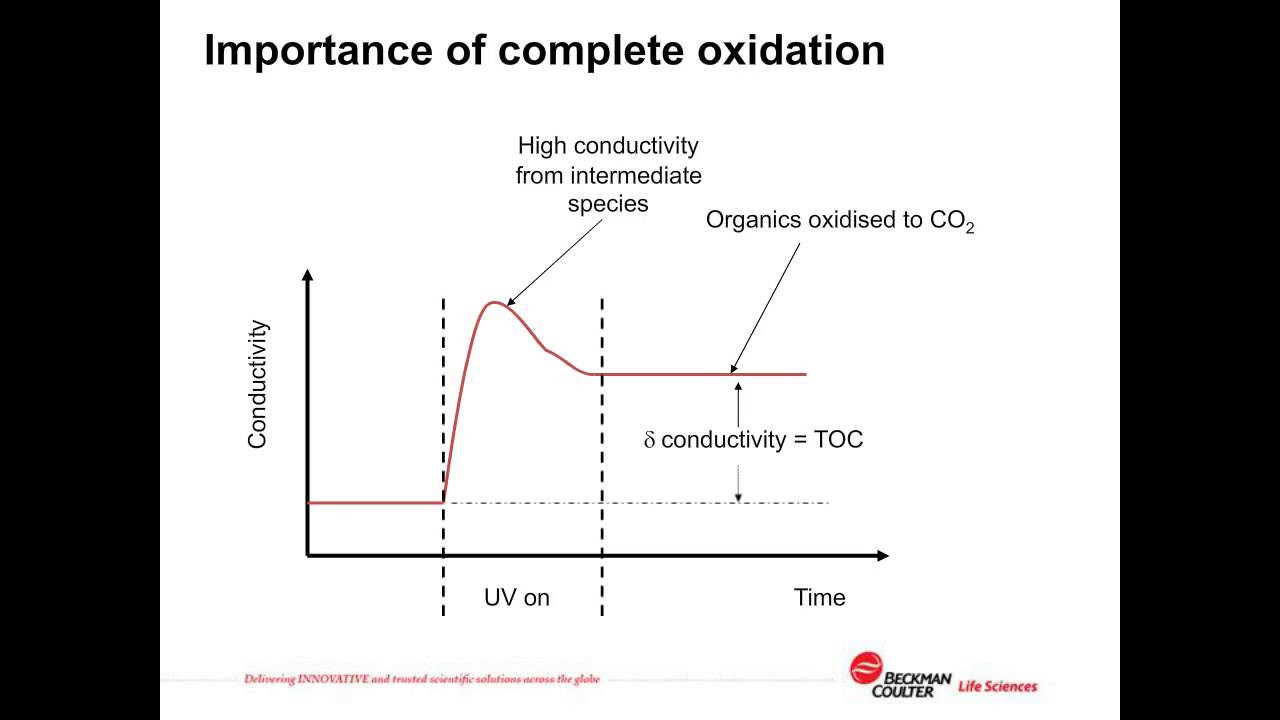
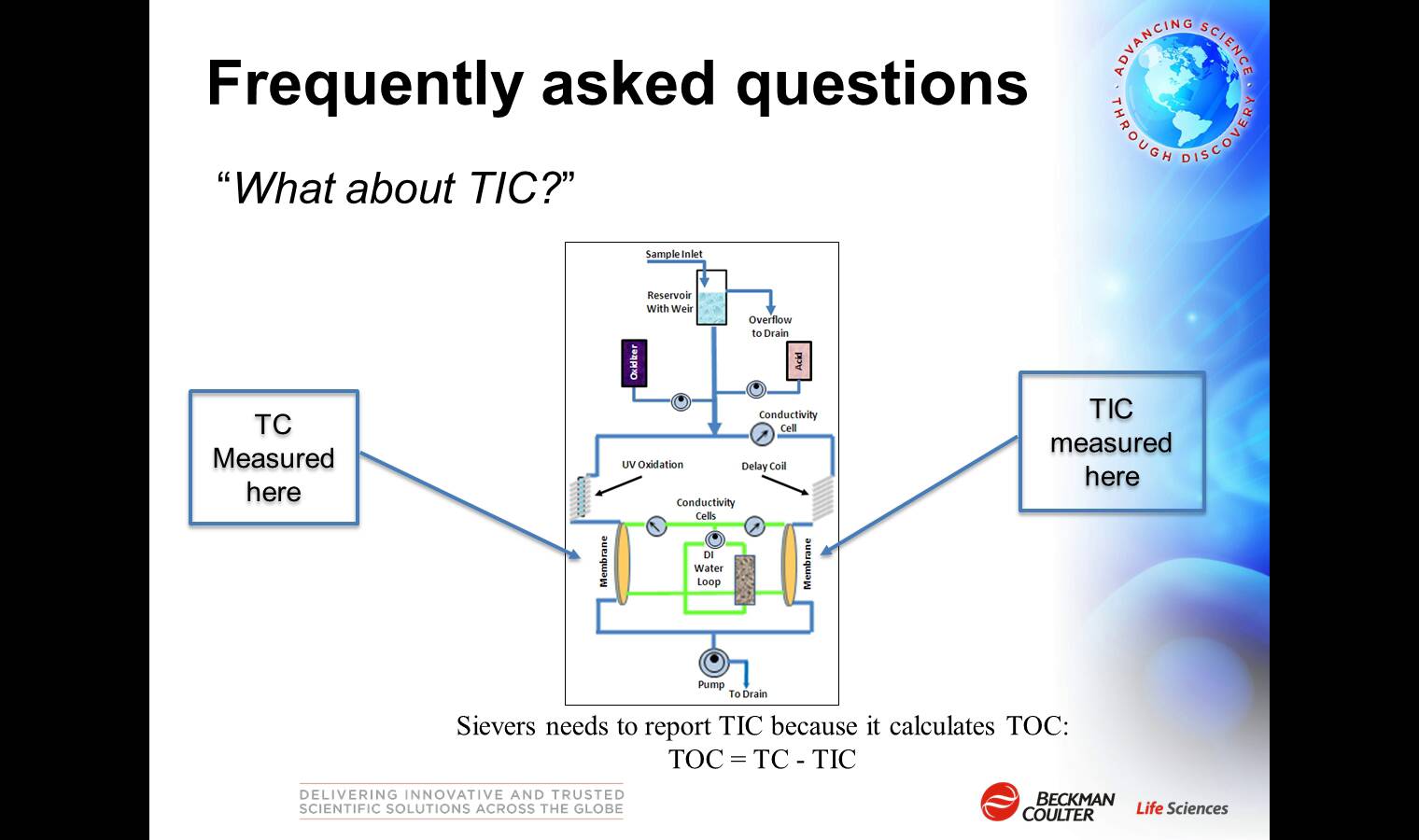
- The importance of the Specificity and Robustness tests for TOC analysis.